Technology
Green Hydrogen and the Challenges for Clean Energy

Hydrogen: A Key Vector in the Energy Transition
Hydrogen will play a crucial role in the global energy mix by 2050. Along with other low-carbon solutions, it will be essential for sectors that are difficult to electrify. Its versatility and potential for production from non-polluting sources make it a key element for the energy transition.
Even in a more limited decarbonization scenario, hydrogen's capacity to store and transport clean energy and reduce emissions makes it especially relevant. Therefore, it is urgent to reduce the cost of green hydrogen production and accelerate its global adoption as a sustainable energy vector.
Clean but Expensive:
The Problems of Green Hydrogen

Is a Practical Alternative?
High Production Cost
Dependence on Subsidies
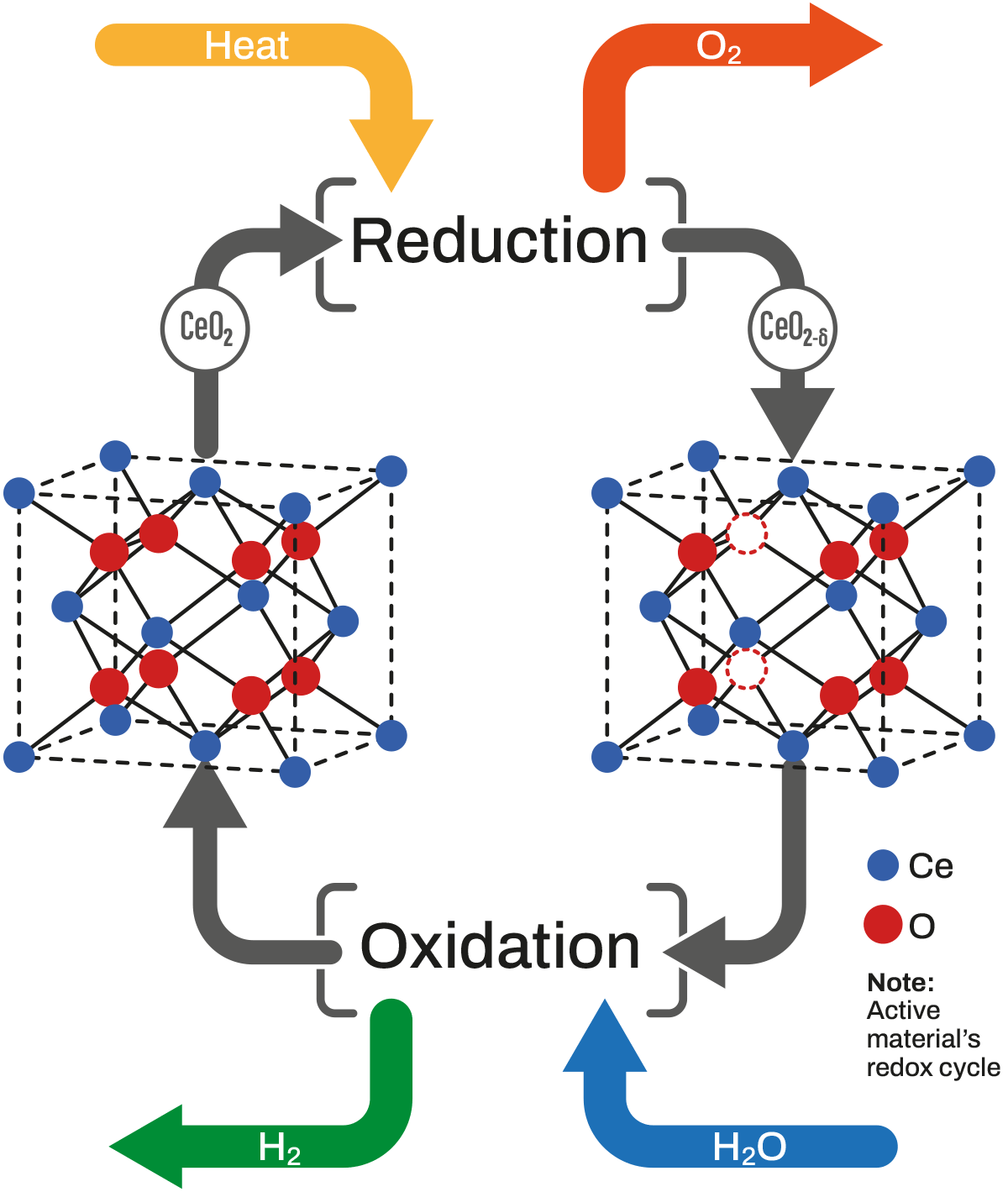
The Hydrolyca Solution: Thermochemical Water Splitting
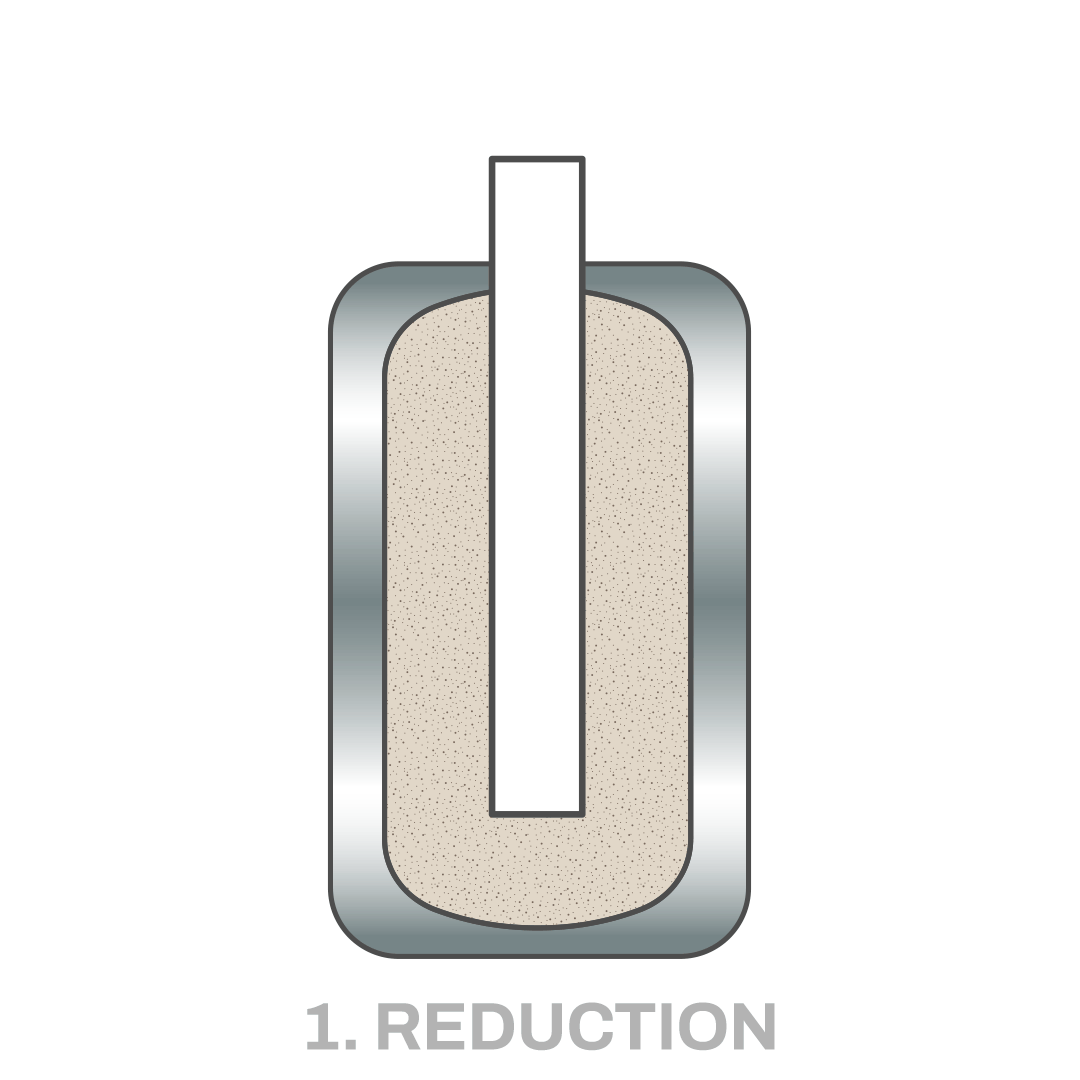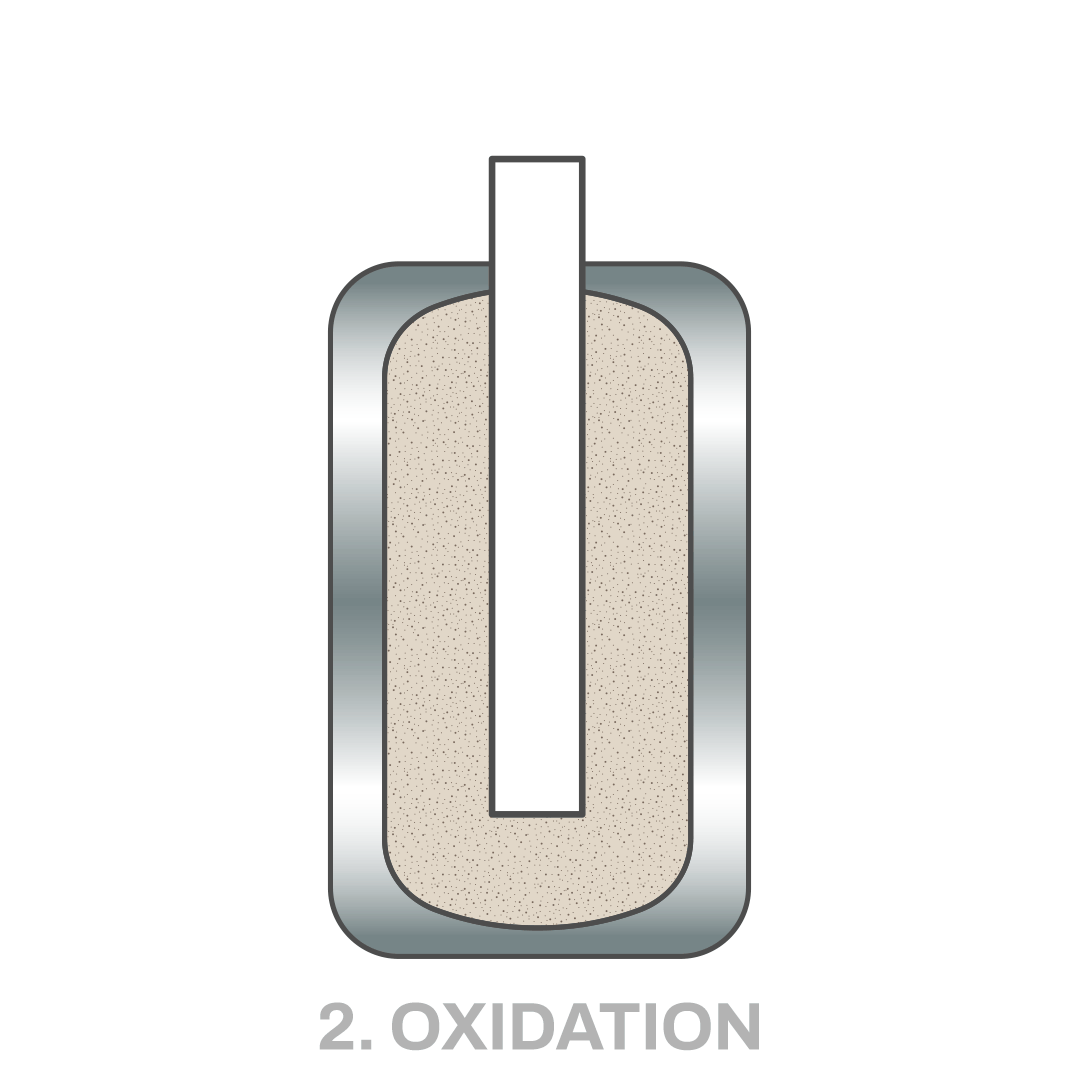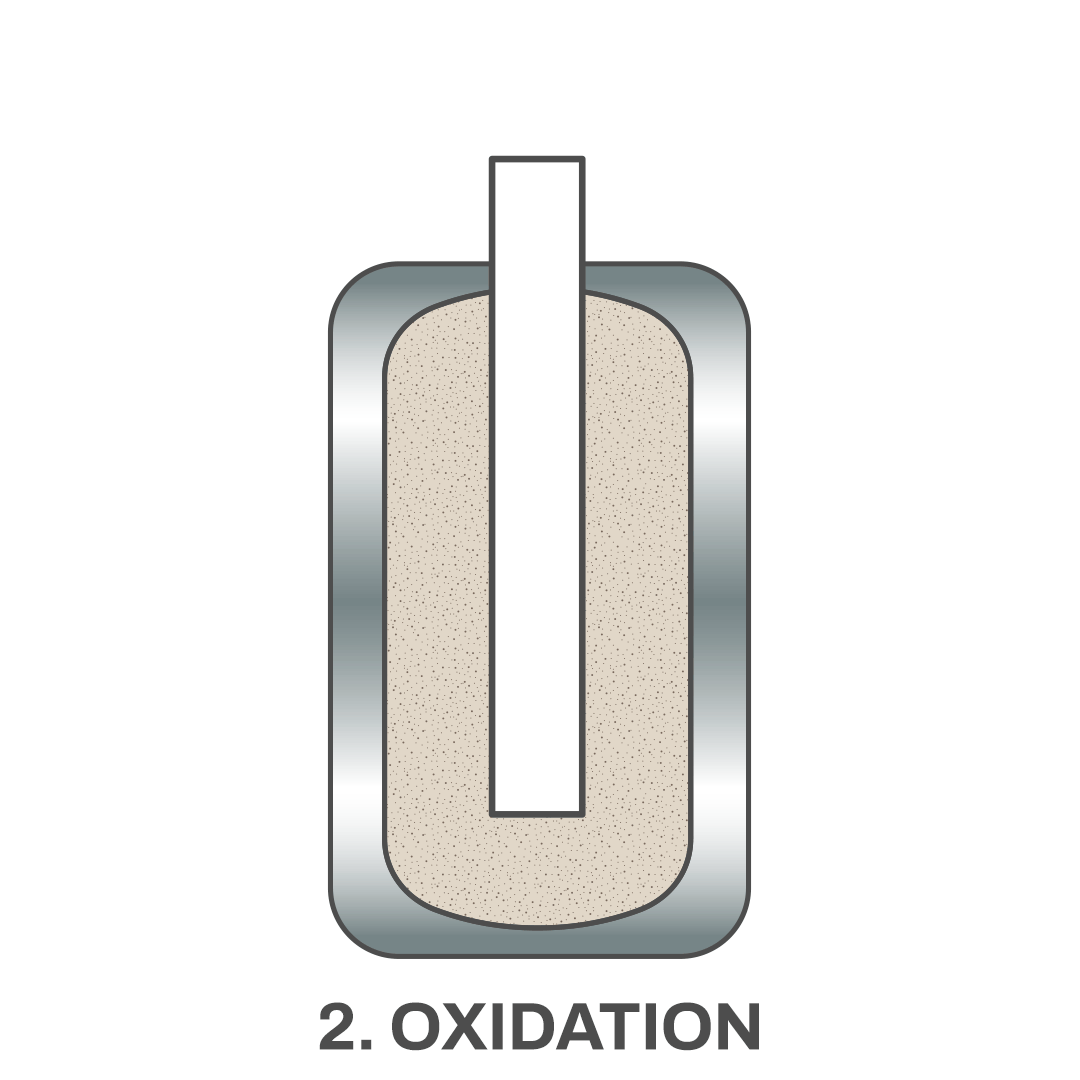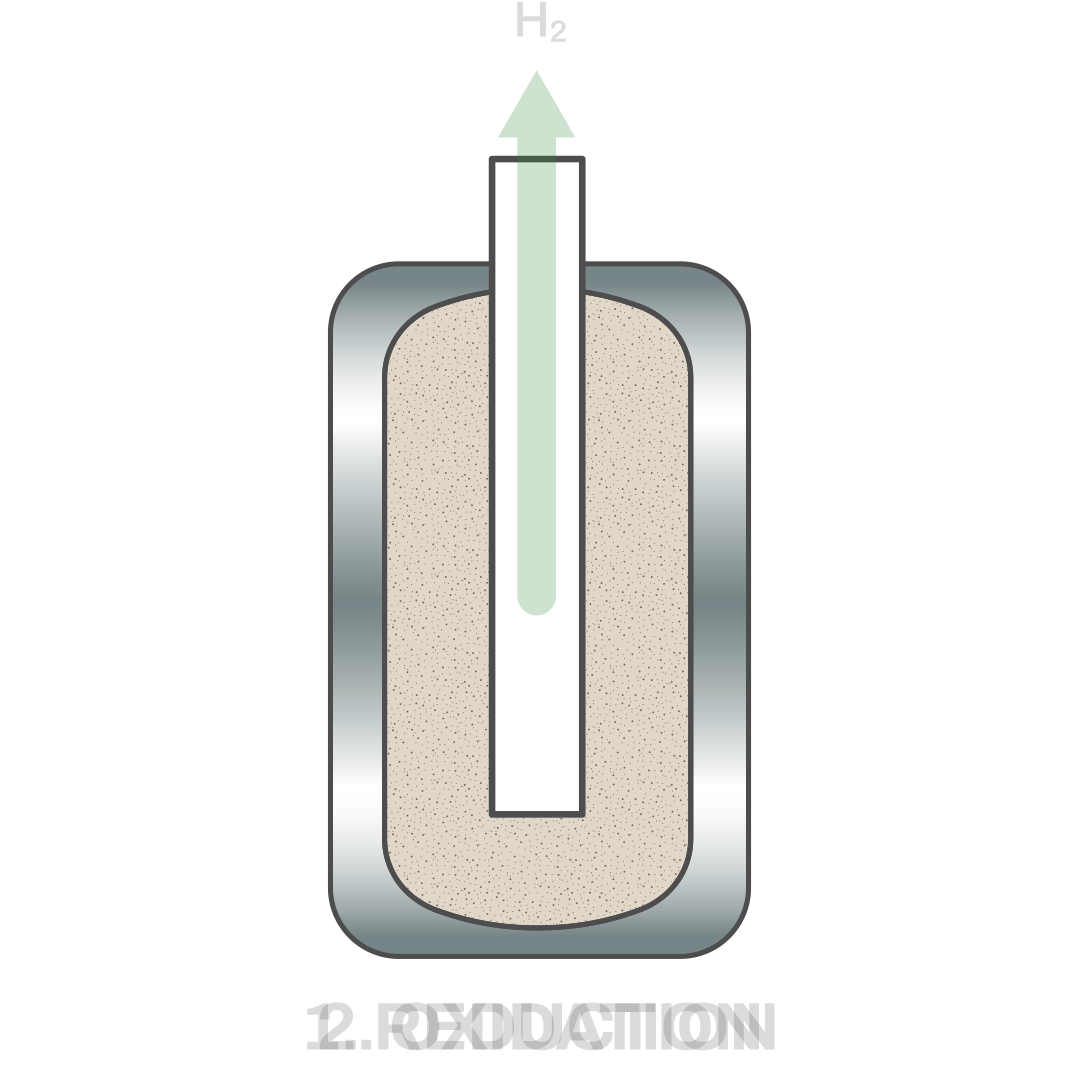
The technology developed by Hydrolyca achieves hydrogen production by dissociating water molecules through thermochemical reactions in quasi isothermal conditions between an active material and water vapor.
A key aspect that distinguishes this method from others is the rapid and brief sequence of redox (reduction-oxidation) cycles. In the first half-reaction, the active material (currently CeO2) is reduced by absorbing heat and releases oxygen. Subsequently, in the second half-reaction, the reduced material reacts with vapor to oxidize again, resulting in hydrogen production.
Reduction: CeO2 + Q ⇆ CeO2-δ + δ/2 O2
Oxidation: CeO2-δ + δ H2O ⇆ CeO2 + δ H2
The intrinsic efficiency of this process is maximized up to 86% when the molar ratio between consumed vapor and produced hydrogen is n = 1.

Hydrolyca´s revolutionary technology enables the production of green hydrogen for less than €1/kg before 2030.
Technology that Makes Hydrogen Green Production Profitable
Hydrolyca's patented technology shifts the equilibrium of the oxidation half-reaction towards hydrogen production while minimizing water consumption. This enables quasi-isothermal cycles, leading to very high efficiency (over 80%).
Hydrolyca's reactor design represents a breakthrough over previous technologies. The company’s patent portfolio currently includes 3 filings, with the first one already published and undergoing PCT application.